Brain BREAK! presents: Tonicity.
Osmosis and diffusion – passive transport – are some of the biggest concepts you will work with in anatomy and physiology. I tell people that if you can get this down, a lot of cells and their jobs will make sense to you. Really cram on transport! It will help you later.
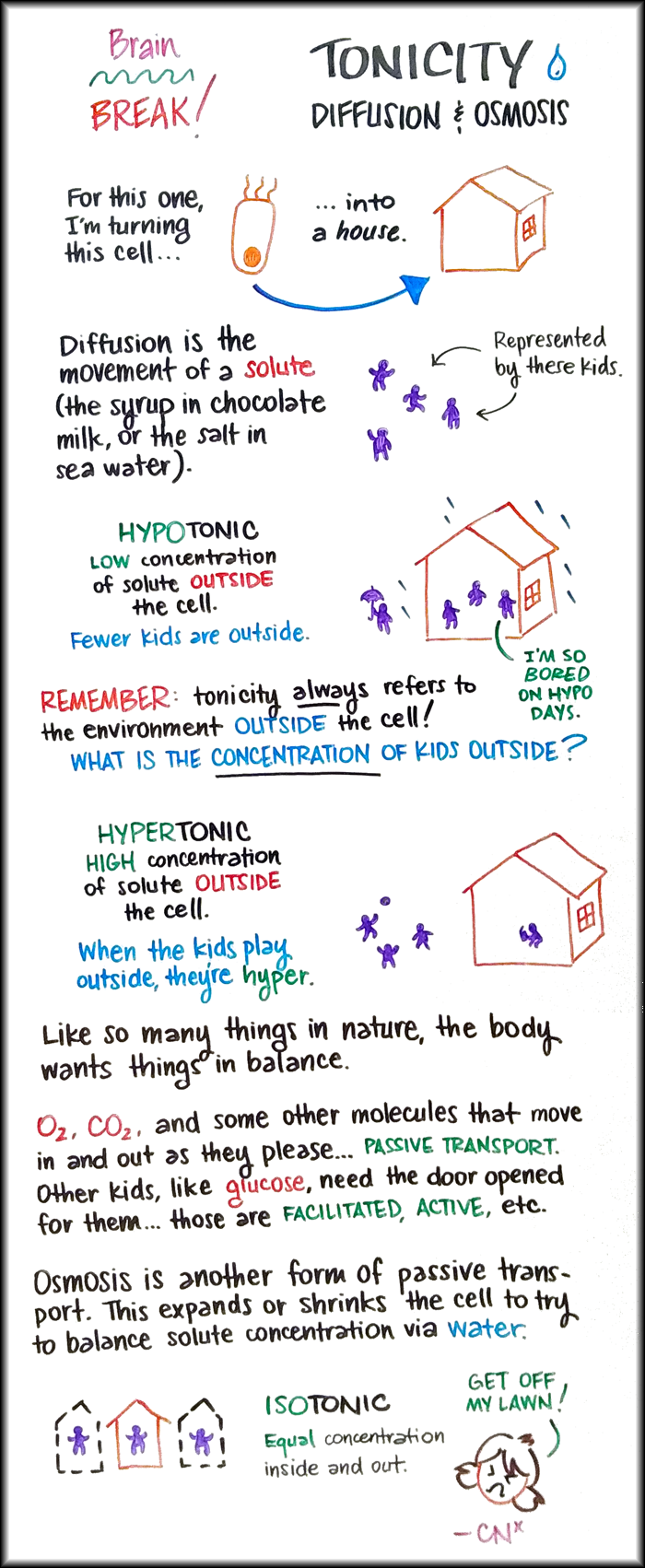
Diffusion is the movement of a solute, like the syrup in chocolate milk, or the salt in sea water, or the urea in pee. When we’re talking about tonicity in this case, we’re talking about the concentration of solutes outside the cell.
Things to plant in your brain:
- The words that describe tonicity (hypo-, hyper-, or iso-) establish the concentration of the solute outside the cell. I really cannot say “outside” enough. If you find yourself thinking, “It’s hypertonic outside, so we could also say that it’s hypotonic inside…” stop yourself! We only care about outside. Don’t overthink it. Look out the window! What is it like outside?
- When we’re assessing tonicity, we’re looking at a moment in time. Look at that selfie (cell-fie?) and sit with the tonicity before your brain tries to problem-solve the whole process. Slow down. Think about which substance is your solute. Compare the concentrations. Think about whether it’s hypo, hyper, or iso outside. Then you can start determining how it got to be this way, what happens next, etc.
With all of that out of the way, I present to you a metaphor: kids represent a solute, and the house represents a cell. There are four kids in this household. Let’s get down to business.
If I tell you, “Today it’s a hypotonic environment,” without even looking at a picture, what can we deduce? Break it down. Hypo means “low.” Kids are our solute. We’re looking outside because that’s how we always describe tonicity. So there is a low concentration of kids outside the house. Imagine a little kid with an umbrella splashing in the puddles all alone.
The next day, it’s a hypertonic environment. Hyper means “high.” Kids are our solute. We’re looking outside as always. You with me still? On a hypertonic day, there is a high concentration of kids outside the house. Imagine three kids romping around in the yard. When the kids are playing outside, they’re hyper. This is how I remember hypertonic.
Lastly, if we have two kids inside and two kids outside, we say it’s isotonic, iso meaning “equal.” This is balance.
It’s important to know that some solutes can come and go through the cell membrane as they please – they’re older kids, like O2 and CO2. They keep things isotonic pretty well on their own. Other solutes are like little kids, who need doors opened for them. Solutes like glucose or salt need help through mediated, active, facilitated, etc. transport types. (What makes a solute one type versus another might depend on the size of the molecule, whether it is chemically polar or nonpolar, and other factors.)
To bring tonicity into a real-world example, sodium chloride will ideally make up 0.9% of the fluid in your body and be isotonic in most cells. That’s 0.9% salt inside a cell and 0.9% outside the cell. Let’s say I am going to drink seawater, which is – besides a really bad idea – about 3.5% sodium chloride. This is going to throw things off in a big way. The moment I drink the seawater, my mouth will become a hypertonic environment – 0.9% in my mouth cells, 3.5% swishing around outside. That’s our snapshot. What will happen next? Let’s talk osmosis.
Osmosis is the other form of passive transport, and it is the great equalizer. Osmosis describes the movement of water and water only. (Think osmosis, oasis.) If the other methods of transport are not keeping things equal, for whatever reason, water will cross the cell membrane to make things equal. What’s important to note is that osmosis will always happen. Sometimes it helps speed up another transport process and sometimes it’s the only thing happening. When it’s the only thing happening, though, that’s intense.
In this case, osmosis can cause the cell to shrink or expand drastically. This is where you see the concepts of a cell bursting (lysis) or shrinking (crenation). Both these types of cell death can happen in lots of different places and cause all kinds of disease. In the case of my drinking seawater, the hypertonic environment of my body will eventually change from my mouth down, one way or another, toward a balance. Because sodium chloride does not diffuse passively through a cell membrane, and other mechanisms don’t want to work, osmosis will cause the cell to spill water into the surrounding environment to even things out, which shrinks cells. Thanks to this water “donation,” I’ll end up with an isotonic concentration of, let’s make up a number, 0.92% sodium chloride. But it’s important to remember that just because the concentration is equal doesn’t mean it’s a good concentration for the body. At a higher concentration of sodium chloride, a lot of my body’s processes will be thrown off, especially by cells that pump salt up and down to do their jobs. That’s why, in many environments, we want isotonic concentrations, and we want the homeostatic concentrations! I’ll need to be rehydrated with some real water tootsweet. Otherwise, the cells of my nerves and kidneys and brain and everything will start to freak out. After my body tries to pee out the salt, I’ll be dehydrated enough that I’ll shrivel into a mummy. (Perhaps a slight exaggeration, but you get the idea.)
If you want to hear more about pathology resulting from osmosis and diffusion, the YouTube channel ChubbyEmu features a doctor describing real-life case studies, many of them illustrating transport mechanics. For example, what happens when someone downs a liter of salty soy sauce?
As for those kids… they need to get off my lawn!
-CNx
