If you are taking a college chemistry class, and you have about $25 USD burning a hole in your pocket, I recommend picking up one of these ball-and-stick molecule building kits. Or the tutoring center in your college probably has one you can use! I bought one (actually, two, because I ran out of oxygens) and used it to build an ATP molecule that I use a couple times a semester when telling students about the magic of cellular respiration. Otherwise, it’s just fun to play with. Having messed around with it before taking biochemistry has given me a huge leg up. I think it’s just because I’ve gotten familiar with some of the geometry and patterns that emerge when building certain molecules.
I’ve mentioned amino acids before. They are these little wads of carbon, nitrogen, oxygen, hydrogen, and other stuff that link up to make proteins. And they’re everywhere. When you pee, a lot of that is the nitrogen from discarded amino acids!
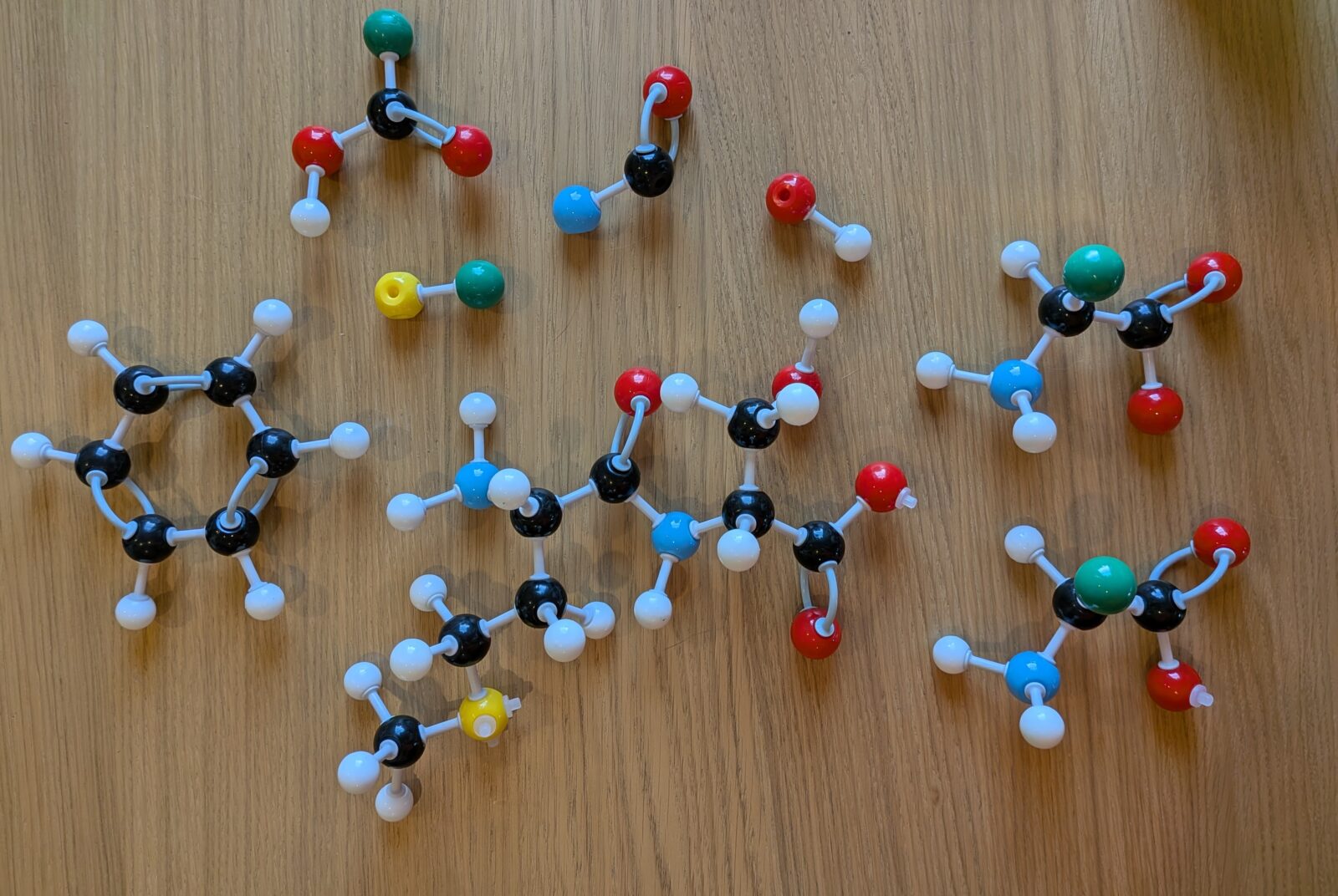
Every amino acid has the same basic skeleton. A nitrogen on one end (blue), a special “alpha” carbon (black), and a carbon (black) with two oxygens (red) tailing off the end. But Cam! What’s that green ball? Well, I’m glad you asked, imaginary person. That’s what I’m using as a placeholder for the “R group.” It branches off of the alpha carbon.

If the skeleton of the amino acid is the cream, milk, and sugar that makes up any ice cream, then the R group is the flavoring. It’s your vanilla bean, cherry, or choco chunk. Your body happens to make use of 20 flavors to build proteins. See, proteins are very fussy molecules with very specialized jobs, so the R groups have very specific chemical traits that make them interact in goofy ways. Sometimes two nearby R groups have sulfur atoms that want to do a special handshake, or one amino acid is repelled by another one because they have the same electrical charges, or nonpolar R groups try to tuck in their little heads to hide from water. These R groups are what make proteins the twisty, bendy bowls of spaghetti that they are. They help your blood cells to cling to oxygen, your spit to break down sugar, and your muscles to contract. Put the wrong amino acids in the wrong place and you might start to have problems.
If a protein is made by putting amino acids together, how do we put amino acids together? Well, that’s easy! With a peptide bond. A peptide is two or more amino acids – nothing too special, necessarily. You might see this reaction called “dehydration synthesis” or “condensation” (they’re kinda the same but different?) because there just so happens to be an oxygen and two hydrogens (H2O) that you can break off to make room for a new bond between the carbon of one and the nitrogen of another. So as you are putting together amino acids, you are also “making” water. Pretty cool, right?

But we’re still looking at this with some green balls that are whimpering, “Cam, we are supposed to be chlorine. What are we doing here?” So let’s get crazy and add some real R groups.

Okay, breathe! Relax! It’s the same thing we’ve been looking at. I’ve circled the R groups for you. Our “flavor” of amino acid on the left is methionine – look at that pretty yellow sulfur – and on the right we have serine – which is a little more demure. Yessir, two amino acids with their respective R groups joined by a peptide bond. If we look at the center of the molecule, we see this nice zigzag backbone forming.
I was just messing around and thought I would share! Some people have been warning me about taking biochemistry, but honestly, I’m pretty geeked about it. Calculating equilibrium concentrations using the quadratic formula in General Chemistry II? No, thank you. Putting together stuff with your hands and finding patterns in pretty pictures? Bring it on.
Stay frosty!
-CNx