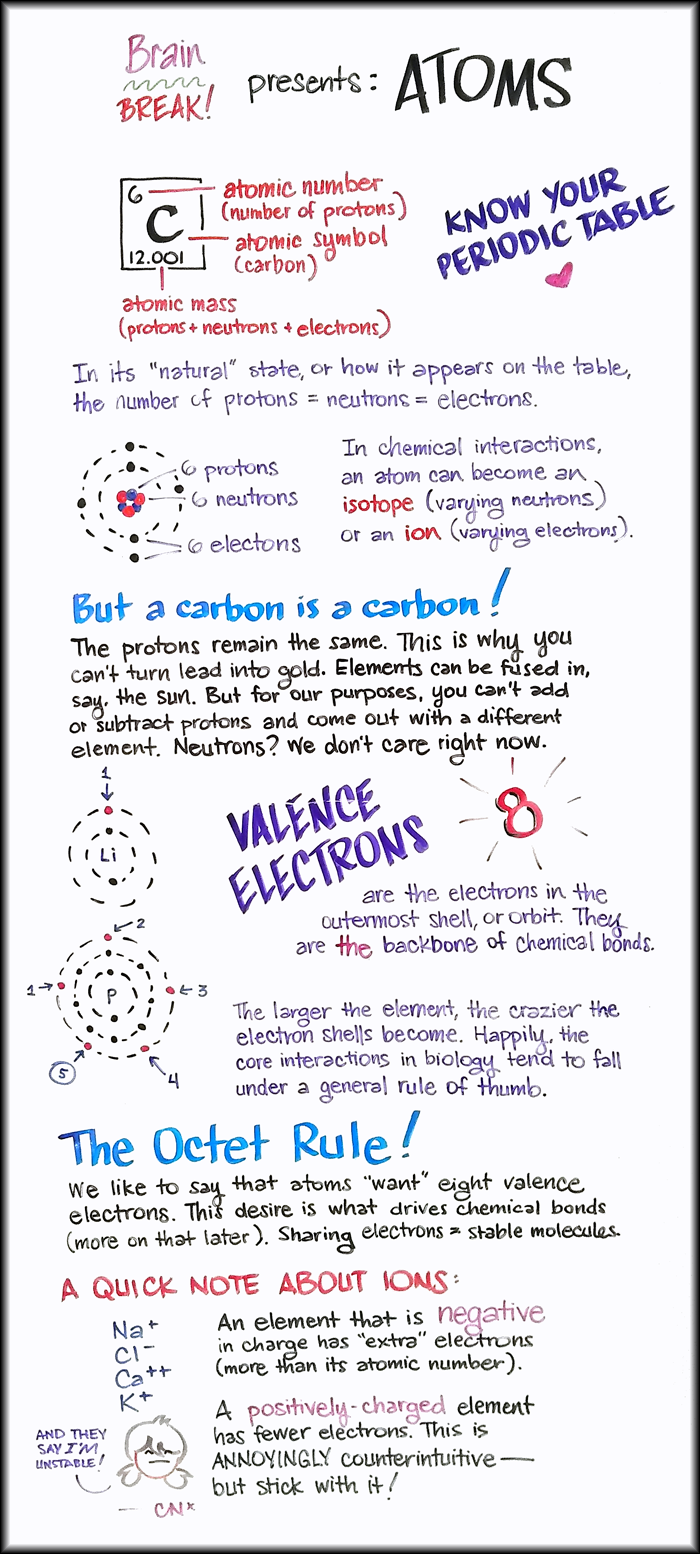
Brain BREAK! presents: Atoms.
Know your periodic table!
C: atomic symbol (carbon)
6: atomic number (number of protons)
12.001: atomic mass (weight of protons + neutrons + electrons)
In its “natural” state, or how it appears on the table, the number of protons = neutrons = electrons.
Carbon, for example, has an atomic number of 6. It contains:
6 protons.
6 neutrons.
6 electrons.
In chemical interactions, an atom can become an isotope (varying neutrons) or an ion (varying electrons.)
But a carbon is s carbon!
The protons remain the same. This is why you can’t turn lead into gold. Elements can be fused in, say, the sun. But for our purposes, you can’t add or subtract protons and come out with a different element. Neutrons? We don’t care right now.
Valence electrons
…are the electrons in the outermost shell or orbit. They are the backbone of chemical bonds.
Lithium has 3 electrons. The inner orbit contains 2 electrons. The outer orbit has 1 electron. This is its 1 valence electron.
Phosphorus has 15 electrons. The inner orbit contains 2 electrons. The next orbit contains 8. The outer orbit has 5 electrons. These are its 5 valence electrons.
The larger the element, the crazier the electron shells become. Happily, the core interactions in biology tend to fall under a general rule of thumb:
The Octet Rule!
We like to say that atoms “want” eight valence electrons. The desire is what drives chemical bonds (more on that later). Sharing electrons equals a stable molecule.
A quick note about ions.
Examples of ions you’ll see in biology:
Na+
Cl-
C++
K+
An element that is negative in charge has “extra” electrons (more than its atomic number). A positively-charged element has fewer electrons. This is annoyingly counter-intuitive, but stick with it!
And they say I’M unstable! -CNx
