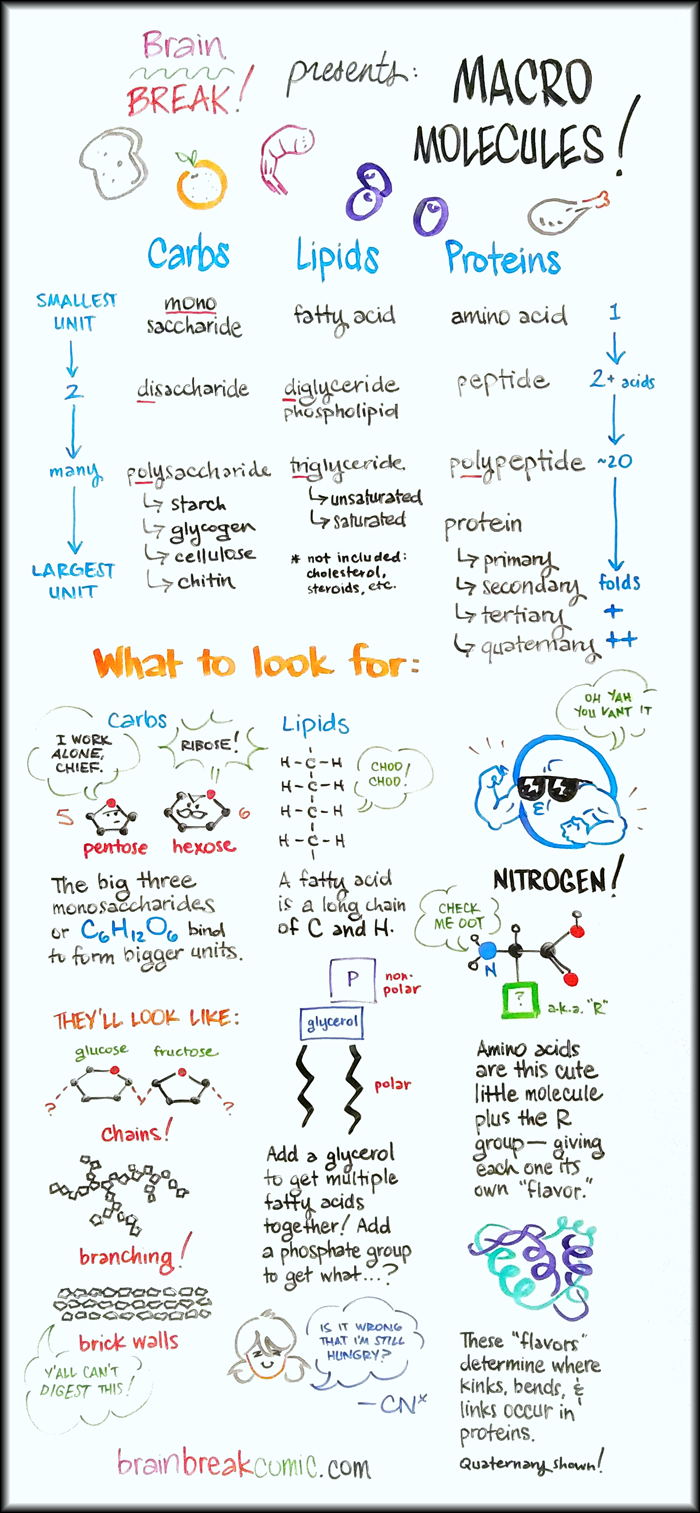
Brain BREAK! presents: Macromolecules.
Here, we’ll “break down” the three major categories of macromolecules from their smallest units, which build upon each other to create larger molecules (the macro part).
Carbohydrates
Smallest unit: Monosaccharide
Two units: Disaccharide
Many: Polysaccharide (e.g. starch, glycogen, cellulose, chitin)
Lipids
Smallest unit: Fatty acid
Two units: Diglyceride, phospholipid
Three: Triglyceride (unsaturated or saturated)
(Not included are cholesterol, steroids, etc. These are types of fats but not “building blocks” that we look at in this topic.)
Proteins
Smallest unit: Amino acid
Two acids or more: Peptide
About 20 acids : Polypeptide
Multiple polypeptides, when folding begins: Proteins – primary, secondary, tertiary, and quaternary.
What to look for?
In sugars, there exist pentagon- (pentose) or hexagon- (hexose) shaped chemical structures of carbon and oxygen, with hydrogen as outliers. In an image, a frowning 5-ringed sugar tells another molecule, “I work alone, Chief.” The six-ringed sugar scolds, “Ribose!”
The three main monosaccharides, or C6H12O6, bind to form bigger units. These include glucose (hexose), galactose (hexose), and fructose (pentose). Put two of these together and you get a disaccharide, for example, lactose. From these building blocks in different combinations, you can make a wide variety of large carbohydrates!
You may see these carbohydrates in chains, branching structures, or “walls.” Cellulose appears as something of a brick wall. It says, “Y’all can’t digest this!” This is why fiber does what fiber does in your diet. And why corn looks pretty much the same coming out as it did going in! Those are some tough polysaccharides.
With lipids, carbons are bonded to each other in a long line, with each carbon carrying hydrogens (one or two, depending – saturated vs. unsaturated fats). One fatty acid unit is this long chain of C and H. Add a glycerol (a “head”) to get multiple fatty acids together. Add a phosphate group to this to get what…?
Proteins? I look for nitrogen! A hulking atom with sunglasses flexes its biceps, saying “Oh yah, you vant it.” Within the X-like structure of an amino acid, this same nitrogen announces, “Check me oot.” Amino acids are a cute little molecule (our star nitrogen, two carbons, two oxygens, and a few hydrogens for good measure) plus what is called an R group – these variations in the R group give each amino acid its own “flavor.” These “flavors” determine where kinks, bends, and links occur in proteins. A quaternary protein model appears as a series of curly scribble-looking forms, for example, hemoglobin.
Is it wrong that I’m still hungry? -CNx

Brilliant! Very fun too.🙂